Research Program Objectives
At the Orthopaedic Translational Engineering Lab (OTEL), our research is focused on advancing clinical solutions through cutting-edge optical imaging and therapeutic technologies. We are committed to accelerating the translation of new technologies, answering foundational questions, equipping the next generation of practitioners, rekindling the entrepreneurial spirit, and improving the life and wellbeing of patients. Our work is organized into three major thrusts:
1. Fluorescence-Guided Debridement
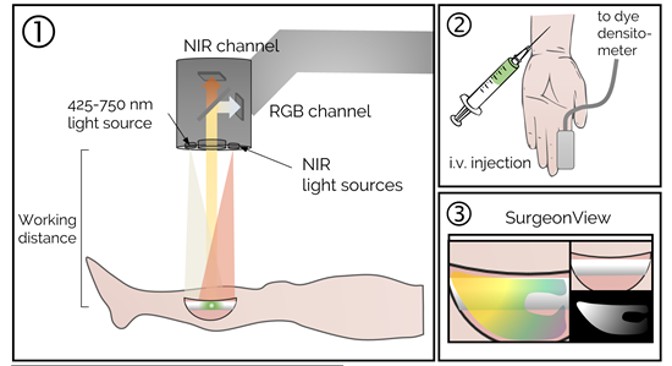
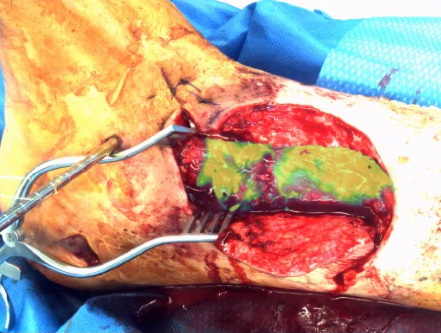
Fluorescence-guided debridement (FGD) is a cornerstone of our research, aimed at improving outcomes in orthopaedic trauma by enabling real-time identification and removal of devitalized and infected tissue. Our lab develops advanced imaging systems and techniques to guide surgeons in managing complex infections and trauma, reducing complications like nonunion and chronic osteomyelitis. Key areas of investigation include:
- Fracture-Associated Infections: Utilizing indocyanine green (ICG) and novel fluorophores to detect biofilm-related infections in open fractures, enhancing debridement accuracy.
- Necrotizing Soft-Tissue Infections: Applying dynamic fluorescence imaging to identify life-threatening infections early, facilitating rapid and precise surgical intervention.
- Bone Perfusion Assessment: Developing real-time perfusion mapping algorithms to assess bone viability during surgery, guiding decisions to preserve healthy tissue.
- System Development: Engineering user-friendly FGD systems with optimized UI/UX for intraoperative use, supporting clinical translation and FDA IDE applications.
Our work has pioneered patient-specific arterial input functions for accurate perfusion assessment and demonstrated FGD’s potential to reduce infection rates in high-energy fractures, with ongoing efforts toward pivotal clinical trials.
Selected Publications
Elliott JT, Henderson E, Streeter SS, Demidov V, Han X, Tang Y, Sottosanti JS, Bateman L, Brůža P, Jiang S, Gitajn IL. "Fluorescence-guided and molecularly-guided debridement: identifying devitalized and infected tissue in orthopaedic trauma." Proceedings of SPIE--the International Society for Optical Engineering, 2023. PMID: 37056956
Ray GS, Streeter SS, Bateman LM, Elliott JT, Henderson ER. "Real-time identification of life-threatening necrotizing soft-tissue infections using indocyanine green fluorescence imaging." Journal of Biomedical Optics, 2024. PMID: 38745983
Tang Y, Jiang S, Sottosanti JS, Usherwood T, Cao X, Bateman LM, Fisher LA, Henderson ER, Gitajn IL, Elliott JT. "Patient-specific arterial input function for accurate perfusion assessment in intraoperative fluorescence imaging." Journal of Biomedical Optics, 2024. PMID: 39247899
2. Precision Medicine and Probe Development
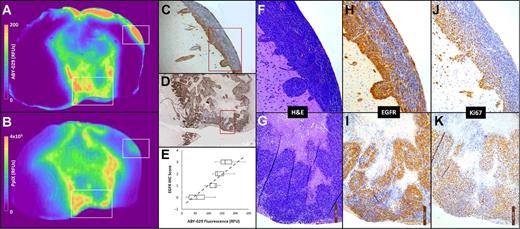
Our lab is advancing precision medicine by developing novel fluorescent probes tailored to specific molecular targets in orthopaedic infections and cancers. This thrust focuses on designing probes, such as those targeting biofilm components or tumor receptors, to enhance intraoperative visualization and personalize treatment. Key areas of investigation include:
- Biofilm-Targeting Fluorophores: Developing imaging probes that target biofilm with low toxicity and high specificity.
- Tumor-Specific Probes: Advancing probes like ABY-029, targeting Epidermal Growth Factor Receptor, for soft-tissue sarcomas and gliomas, improving resection accuracy.
- Clinical Translation: Conducting first-in-human studies to validate probe efficacy, integrating findings into clinical workflows for personalized surgical strategies.
Our probe development efforts have led to breakthroughs in molecular-guided surgery, with applications in both orthopaedics and oncology, supported by robust computational and preclinical validation.
Selected Publications
Samkoe KS, Sardar HS, Gunn JR, Elliott JT, Mansur S, Feldwisch J, Pogue BW, Linos K, Paulsen KD, Henderson ER. "First-in-human Study of ABY-029, a Novel Fluorescent Peptide that Targets Epidermal Growth Factor Receptor, Applied to Soft-Tissue Sarcomas." Molecular Cancer Therapeutics, 2024. PMID: 39686611
Elliott JT, Dsouza AV, Davis SC, Olson JD, Paulsen KD, Roberts DW, Pogue BW. "Review of fluorescence guided surgery visualization and overlay techniques." Biomedical Optics Express, 2015. PMCID: PMC4605037
Elliott JT, Marra K, Evans LT, Davis SC, Samkoe KS, Feldwisch J, Paulsen KD, Roberts DW, Pogue BW. "Simultaneous in vivo fluorescent markers for perfusion, protoporphyrin metabolism, and EGFR expression for optically guided identification of orthotopic glioma." Clinical Cancer Research, 2017. PMCID: https://doi.org/10.1158/1078-0432.CCR-16-1400
3. Antimicrobial Photodynamic Therapy
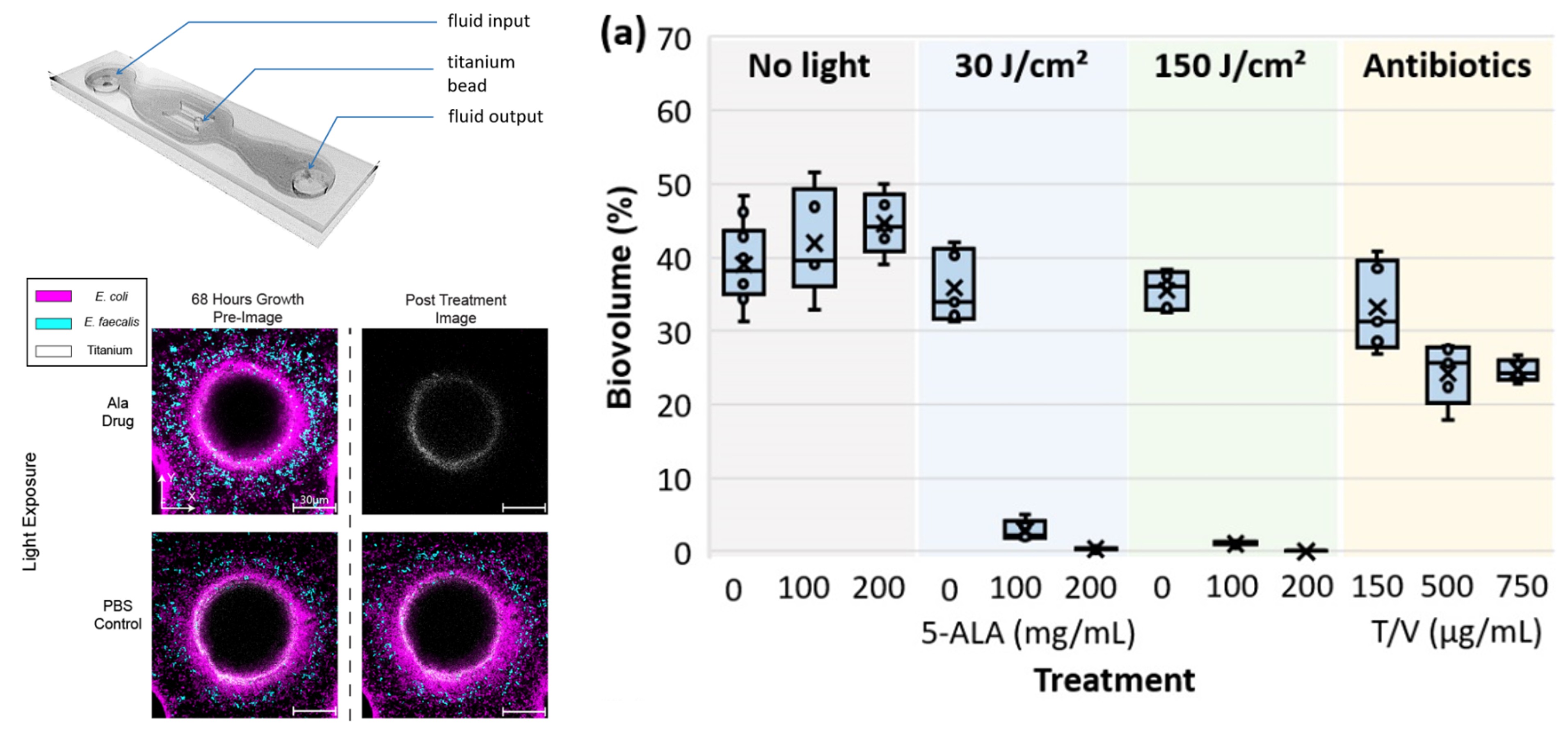
Antimicrobial photodynamic therapy (aPDT) leverages light-activated agents to eradicate bacteria, including those within biofilms, offering a novel approach to infection management in orthopaedics. Our lab is developing aPDT protocols to treat contaminated fractures and enhance osseointegrated prostheses, reducing infection-related complications. Key areas of investigation include:
- High-Energy Fracture Infections: Testing aPDT against MRSA and mixed-species biofilms in open fracture models, achieving synergistic effects with antibiotics.
- Osseointegration Prostheses: Applying aPDT to prevent and treat infections at the bone-implant interface, improving prosthesis longevity.
- Therapy Optimization: Using optical coherence tomography (OCT) to assess aPDT efficacy, guiding treatment protocols for clinical translation.
- Combination Therapies: Exploring aPDT combined with antibiotics to achieve complete bacterial suppression, addressing incomplete eradication challenges.
Our aPDT research, supported by R01 AR081952, has demonstrated significant reductions in bacterial bioburden, with preclinical models paving the way for clinical applications in infection control.
Selected Publications
Demidov VV, Bond MC, Demidova N, Gitajn IL, Nadell CD, Elliott JT. "Assessment of photodynamic therapy efficacy against Escherichia coli-Enterococcus faecalis biofilms using optical coherence tomography." Journal of Biomedical Optics, 2025. PMID: 40083371
Rivet C, Elliott JT, Gunn JR, Sottosanti JS, Fearing BV, Hsu JR, Gitajn IL. "Rabbit model of a biofilm-contaminated, percutaneous orthopaedic endoprosthesis." OTA International: The Open Access Journal of Orthopaedic Trauma, 2025. PMID: 40071171
4. Tracer Kinetics and Drug Receptor Imaging
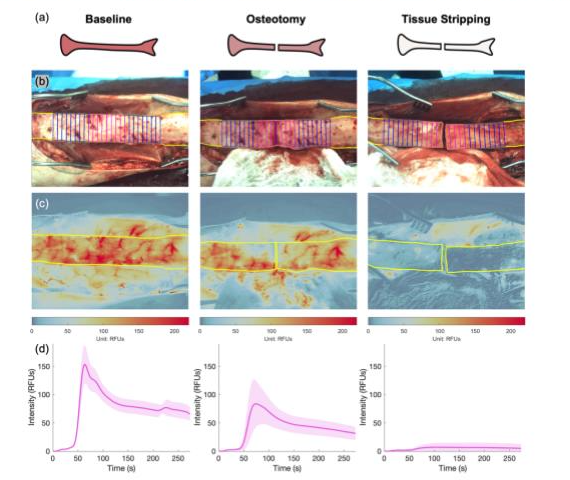
Our research in tracer kinetics and drug receptor imaging focuses on quantifying the uptake and binding of optical tracers to improve drug delivery and treatment outcomes. By developing novel kinetic models and imaging techniques, we enhance the understanding of molecular interactions in orthopaedics and oncology. Key areas of investigation include:
- Arterial Input Function (AIF): Creating non-invasive methods, such as modified pulse oximetry, to characterize AIF for accurate perfusion and tracer uptake measurements.
- Bone and Tumor Perfusion: Applying kinetic models to measure periosteal and endosteal blood flow, guiding surgical and therapeutic decisions.
- Receptor Binding: Imaging opioid and EGFR receptor kinetics to assess drug biodistribution, with applications in pain management and cancer therapy.
- Preclinical Validation: Using large animal models to validate tracer kinetics, ensuring translatability to human clinical studies.
Our work has advanced quantitative fluorescence imaging, with applications in intraoperative perfusion assessment and drug receptor studies, supported by innovative AIF methodologies.
Selected Publications
Elliott JT, Jiang S, Pogue BW, Gitajn IL. "Bone-specific kinetic model to quantify periosteal and endosteal blood flow using indocyanine green in fluorescence guided orthopedic surgery." Journal of Biophotonics, 2019. PMCID: 30963727
Elliott JT, Tichauer KM, Samkoe KS, Gunn JR, Sexton KJ, Pogue BW. "Direct characterization of tracer plasma curves by fluorescence imaging of exposed carotid artery to facilitate kinetic analysis." Molecular Imaging and Biology, 2014. PMCID: DOI:10.1007/s11307-013-0715-y
Tang Y, Jiang S, Sottosanti JS, Usherwood T, Cao X, Bateman LM, Fisher LA, Henderson ER, Gitajn IL, Elliott JT. "Patient-specific arterial input function for accurate perfusion assessment in intraoperative fluorescence imaging." Journal of Biomedical Optics, 2024. PMCID: PMC11379448
5. Foundational Biofilm Research and Novel Imaging Strategies
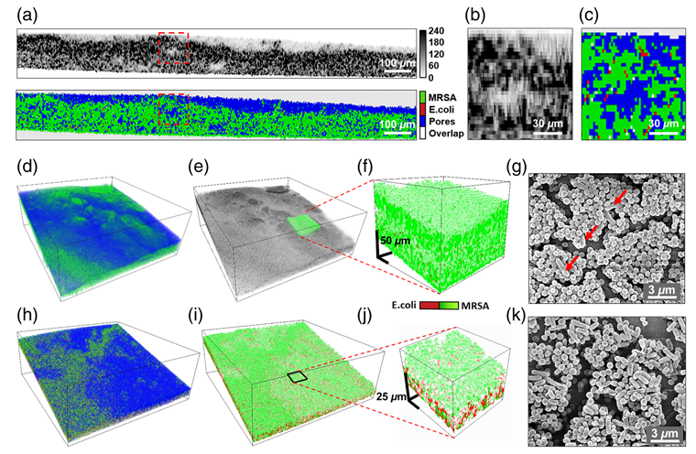
Biofilms are a major challenge in orthopaedics, driving persistent infections and implant failures. Our lab conducts foundational research to understand biofilm dynamics and develops novel imaging strategies to detect and manage these infections. This thrust integrates preclinical models with advanced optical techniques to advance infection control. Key areas of investigation include:
- Preclinical Models: Developing high-energy fracture and implant models with MRSA and mixed-species biofilms to study infection progression and treatment efficacy.
- Optical Coherence Tomography (OCT): Using OCT to non-invasively assess biofilm structure and density, providing insights into therapeutic outcomes.
- Longitudinal Imaging: Employing micro- and macrofluidic devices to monitor biofilm growth over time, enabling dynamic studies of bacterial behavior.
- Novel Fluorophores: Designing fluorophores to visualize biofilms, such as those targeting eDNA or matrix proteins, for intraoperative detection.
Our research has advanced biofilm imaging through OCT and fluorescence techniques, with preclinical models supporting the development of targeted therapies for implant-associated infections.
Selected Publications
Demidov VV, Bond MC, Demidova N, Gitajn IL, Nadell CD, Elliott JT. "Assessment of photodynamic therapy efficacy against Escherichia coli-Enterococcus faecalis biofilms using optical coherence tomography." Journal of Biomedical Optics, 2025. PMID: 40083371
Demidov VV, Clark MA, Streeter SS, Sottosanti JS, Gitajn IL, Elliott JT. "High-energy open-fracture model with initial experience of fluorescence-guided bone perfusion assessment." Journal of Orthopaedic Research, 2023. PMID: 36192829
Han X, Demidov V, Vaze VS, Jiang S, Gitajn IL, Elliott JT. "Spatial and temporal patterns in dynamic-contrast enhanced intraoperative fluorescence imaging enable classification of bone perfusion in patients undergoing leg amputation." Biomedical Optics Express, 2022. PMID: 35781962
Our Partners
Our Current and Past Funding Providers










